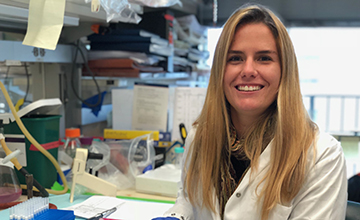
“My focus is to better understand the role that Cullin-3-related protein degradation may play in the pathological mechanism leading to the development of nemaline myopathy,” Jordan Blondelle says. “It’s important because the underlying mechanisms of this life-threatening disease currently are poorly understood.”
Jordan Blondelle, postdoctoral researcher at the University of California San Diego in La Jolla, was awarded an MDA development grant totaling $180,000 over three years to increase understanding of the underlying mechanisms in nemaline myopathy.
Muscle mass maintenance is tightly regulated by a balance between muscle growth and atrophy, reflecting the balance between protein synthesis and degradation at the cellular level. Accumulation of misfolded proteins or premature degradation of proteins is often associated with diseases such as skeletal muscle myopathies.
The degradation of muscle proteins is accomplished mainly through a cellular “clean-up and disposal system” called the ubiquitin-proteasome system, or UPS. The Cullin family of proteins, a key component of the UPS system, incorporate into complexes after which they are able to bind and mark unwanted proteins for degradation. Recently, mutations in several binding partners of Cullin-3, a Cullin protein family member, were found in people with severe forms of nemaline myopathy. These findings suggest a specific and important function for Cullin-3 targeted protein degradation during muscle development and maintenance.
Using mutant mice in which Cullin-3 is depleted in muscles, Blondelle and colleagues determined that the protein is necessary for survival, and will now look at how the loss of Cullin-3 affects muscle development, structure and function.
The team expects to find new target proteins of the Cullin-3 complex that would be misregulated in the absence of Cullin-3 and responsible for the myopathy observed in mice. In addition, the team intends to decipher, for the first time, how protein degradation mediated by Cullin-3 is relevant for muscle physiology, and to provide insight into the pathological mechanisms leading to nemaline myopathy in people.
If successful, Blondelle’s work could inform the development of therapies for nemaline myopathy.
Grantee: Nemaline Myopathy – Jordan Blondelle, Ph.D.
Grant type: Development Grant
Award total:
Institution:
Country: