Search our Grants
MDA’s research program awards grants to the world’s best scientists investigating promising theories and therapies that may accelerate treatments and cures for families living with muscular dystrophy, ALS and related neuromuscular diseases.
Grant - Summer 2011 - ALS — Jasna Kriz, Ph.D.
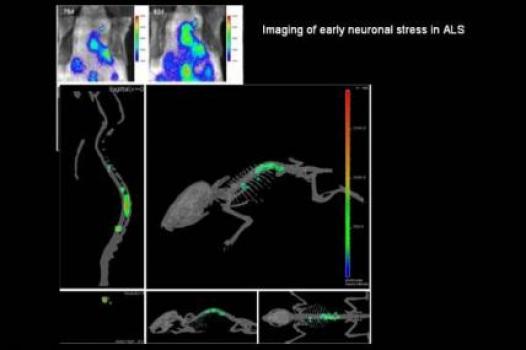
Jasna Kriz, associate professor in the department of psychiatry and neuroscience at Laval University, Quebec City, Canada, was awarded an MDA research grant totaling $445,086 over a period of three years to help refine and describe a new mouse model that will enable scientists to visualize different aspects of the disease process in ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease).
Previously, Kriz and colleagues developed mouse models with bioluminescent and fluorescent genes (from fireflies) that allow researchers to visualize ALS-related events such as neuroinflammation and neuronal damage in the brains and spinal cords of living mice. They even were able to detect distinct and disease-specific signals linked to presymptomatic stages of the disease.
Now, Kriz plans to use the previously generated models to generate ALS imaging reporter mice, which will enable scientists to visualize, in live mice, different elements of the ALS disease process.
Once their characteristics are fully understood, the ALS reporter mice will represent a unique tool that will allow scientists to study the ALS disease process and real-time response to experimental therapies.
Favorable results are expected to lead to more efficient translation of experimental therapies to the clinic and new therapeutic strategies for ALS.
Funding for this MDA grant began August 1, 2011.
Grantee: ALS — Jasna Kriz, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Winter 2012 - Muscle Physiology - Chris Weihl, M.D., Ph.D.

Chris Weihl, assistant professor of neurology at Washington University School of Medicine in St. Louis, was awarded an MDA grant totaling $397,064 over a period of three years. The funds will help support Weihl’s research into a process called autophagy in skeletal muscle. Data gleaned from Weihl’s studies may be applicable to a number of neuromuscular disorders including amyotrophic lateral sclerosis (ALS) and the muscular dystrophies; Weihl and colleagues will conduct their studies on a mouse model of myofibrillar myopathy (MFM).
Autophagy, which means "self-digestion," is a cellular cleanup and garbage-disposal system. Cells use it to degrade and destroy abnormal cellular or protein components that otherwise could lead to toxicity and cell death.
One hallmark in a number of neuromuscular diseases is the presence of protein clumps called aggregates or inclusions in affected tissues. It’s unknown whether facilitating the clearance or degradation of these inclusions is beneficial.
Weihl and colleagues plan to test FDA-approved drugs reported to enhance autophagy to determine whether protein degradation via autophagy is protective. The group will test the drugs in a newly developed mouse model of myofibrillar myopathy.
“These studies will answer the question of whether enhancing autophagy in protein-aggregate disorders is protective,” Weihl said.
Funding for this MDA grant began February 1, 2012.
Grantee: Muscle Physiology - Chris Weihl, M.D., Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2011 - ALS — Don Cleveland, Ph.D.

MDA has awarded a research grant totaling $429,983 over three years to Don Cleveland, departmental chair of cellular and molecular medicine; professor of medicine, neurosciences, and cellular and molecular medicine; and member of the Ludwig Institute for Cancer Research in La Jolla, Calif. The funds will help support Cleveland’s research into the connection between mitochondria and ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease).
Mitochondria, the intracellular compartments that consume oxygen to produce chemical fuel that supports the cell, have been implicated as targets for toxicity in the inherited, SOD1-associated forms of ALS.
It is not known, however, in which cell types of the nervous system mitochondrial damage occurs, whether the ensuing mitochondrial dysfunctions are a cause or a consequence of neuronal degeneration during the disease and if the effects are the same in SOD1-associated ALS caused by different SOD1 mutations.
Using mice that are genetic mimics of inherited, SOD1-related ALS, Cleveland intends to determine at what disease stage and in which cell types of the central nervous system mitochondrial dysfunction occurs, and whether rescue of individual mitochondrial functions or an increase in mitochondrial activity may alter the ALS disease course.
“This comparative analysis will, in principle, provide a test for whether mitochondria are a common target of toxicity,” Cleveland said.
Funding for this MDA grant began August 1, 2011.
Grantee: ALS — Don Cleveland, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Winter 2012 - MMD - Mani Mahadevan, M.D.

Mani Mahadevan, a professor at the University of Virginia in Charlottesville was awarded an MDA grant totaling $281,352 over a period of two years. The funds will help support Mahadevan's investigation into potential therapies for type 1 myotonic muscular dystrophy (MMD1, or DM1).
A key component of Mahadevan's new work is collaboration between the Mahadevan lab and Novartis through the Genomics Institute of the Novartis Research Foundation. Together, the two groups intend to identify compounds that correct defects in muscle tissue formation in DM1.
They will do this via a screening method that will evaluate approximately 850,000 compounds; promising candidates will then be evaluated in a mouse model to determine whether they lead to any improvement in the generation of mature muscles in DM1.
Compounds identified through the screening process may be used as starting points for further development of a DM1 therapy.
"The current state of translational research is very exciting," Mahadevan said. "A number of different approaches, including antisense oligos and small molecules are being developed and tested by labs around the world to try to develop therapies for myotonic dystrophy and have yielded promising results in mouse models."
Funding for this MDA grant began February 1, 2012.
Grantee: MMD - Mani Mahadevan, M.D.
Grant type:
Award total:
Institution:
Country:
Grant - Spring 2011 - ALS — Clotilde Lagier-Tourenne, M.D., Ph.D.

Clotilde Lagier-Tourenne, a postdoctoral fellow at the University of California, San Diego, in La Jolla, was awarded an MDA development grant totaling $180,000 over a period of three years to study the roles of two proteins, TDP43 and FUS, in ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease).
ALS-causing mutations in the genes for two RNA binding proteins, TDP43 and FUS, appear to cause disruption in the processing of RNA (the chemical step that directs protein synthesis).
In a research mouse model, Lagier-Tourenne and colleagues have identified RNAs that TDP43 normally binds to, as well as abnormalities in the processing of RNA that occur when TDP43 is depleted.
Similarly, in their new work, the researchers plan to determine the role of FUS in the regulation of RNA in the mouse central nervous system.
The team's proposed set of studies is expected to identify a set of alterations to normal RNA processing that will define a TDP43- and FUS-dependent ALS disease signature.
"I am really grateful for the support from MDA," Lagier-Tourenne said. "It represents strong encouragement at this stage of my career and will allow me to extend my research on the role of RNA processing in neurodegeneration."
Funding for this MDA grant began August 1, 2011.
Grantee: ALS — Clotilde Lagier-Tourenne, M.D., Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Winter 2012 - MMD - Laura Ranum, Ph.D.

MDA awarded a research grant totaling $415,092 over a period of three years to Laura Ranum, professor of molecular genetics and microbiology at the University of Florida in Gainesville.
The funds will help support Ranum's research into the role of a phenomenon called Repeat Associated Non-ATG translation (RAN translation) in myotonic dystrophy (MMD, or DM1).
Ranum and colleagues have discovered a new mechanism, RAN translation, by which repeat sequences (series of repeated segments of DNA) direct protein synthesis in the absence of normal regulatory signals. Evidence suggests that RAN translation results in the production of unexpected mutant proteins in myotonic dystrophy.
In her new work, Ranum and colleagues plan to determine how many of these unexpected proteins are made in myotonic dystrophy, which cells in the body are able to make them, and what effects they have on the disease.
"We have demonstrated that RAN translation occurs in cell culture and in animal models of type 1 myotonic dystrophy, and in tissues from human patients," Ranum said. "We must now consider the effects that these newly discovered mutant proteins could have on the disease."
Funding for this MDA grant began February 1, 2012.
Grantee: MMD - Laura Ranum, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2012 - MG — Lin Mei, M.D., Ph.D.

MDA awarded a research grant totaling $390,000 over three years to Lin Mei, professor and director of the Institute of Molecular Medicine and Genetics at the Medical College of Georgia, part of Georgia Regents University. The funds will help support Mei’s research on the role of a protein called LRP4 in myasthenia gravis (MG).
MG is an autoimmune disease — a disease that occurs when the immune system attacks the body's own tissues. The attack occurs at theneuromuscular junction (NMJ), the space where signals pass between muscle and nerve.
Symptoms include weakness in muscles that control the eyes, face, neck and limbs; partial paralysis of eye movements; double vision and droopy eyelids; and weakness and fatigue in the neck and jaws with problems in chewing, swallowing and holding up the head.
Most MG occurs when the immune system uses special proteins calledantibodies to target either the acetylcholine (ACh) receptor, or muscle-specific kinase (MuSK), a protein that helps organize ACh receptors on the muscle cell surface. But approximately 10 percent of people with the disease are seronegative for antibodies to the ACh receptor or MuSK, meaning the antibodies aren't detectable in their blood.
In preliminary studies, Mei and colleagues have identified an antibody against a protein called LRP4 in people with seronegative MG. (LRP4 is known to play a role in formation and maintenance of the NMJ.)
Now the group is studying the possible role and mechanisms of LRP4 in seronegative MG.
"Such information should contribute to a better understanding of seronegative MG and development of novel diagnostic and therapeutic means for this devastating disease," Mei says.
Funding for this MDA grant began Aug. 1, 2012.
Grantee: MG — Lin Mei, M.D., Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2013 - FSHD — Gregory Block, Ph.D.
Gregory Block, senior fellow in pediatrics at the University of Washington in Seattle, was awarded an MDA development grant totaling $179,994 over a period of three years to search for treatment targets infacioscapulohumeral muscular dystrophy (FSHD).
FSHD occurs when a section at the tip of one chromosome, chromosome number 4, is shortened. When that happens, it allows a gene called DUX4 to remain active into adulthood, instead of becoming inactive after early development. The symptoms of FSHD are due to this extended activity of DUX4, so preventing that activity, or blocking its effects, could be therapeutic in the disease. Block has developed a cell-based system to study DUX4 activation, and has found one chemical pathway in muscle cells that can prevent activation. He will continue investigating this pathway to determine if it can be used to develop treatments for FSHD.
“This is an exciting time for FSHD research,” Block says. “Four years ago, there was still considerable debate as to the genetic cause of the disease, and today we are challenged with defining pragmatic approaches for disease intervention. We are hoping the data generated from this project will provide considerable insight into the mechanisms used by cells to silence DUX4.”
Funding for this MDA grant began August 1, 2013.
Grantee: FSHD — Gregory Block, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Winter 2012 - MG - Premkumar Christadoss, M.B.B.S.

Premkumar Christadoss, a professor in the department of microbiology and immunology at the University of Texas Medical Branch in Galveston, was awarded an MDA research grant totaling $390,000 over a period of three years to study the potential for gene therapy as a treatment in myasthenia gravis (MG).
In MG, an "autoimmune" disease, the immune system attacks the body's own tissues. The attack occurs at the junction between nerve and muscle, and targets the acetylcholine receptor, the part of a muscle cell that receives signals from a nerve cell. Specific functions involved in driving the attack are generated by components (including the proteins C2, C4 and C1) in what is known as the complement system.
In a research mouse model of MG, Christadoss and colleagues will administer what are called small interfering RNAs (siRNAs) designed to block activation of C2, C4 and/or C1. Inhibition of the proteins should shed light on each protein's role in the autoimmune disease process and guide the development of therapies that can target them.
"Successful completion of this project will lead to human complement C2, C4 or C1 siRNA gene therapy for myasthenia gravis," Christadoss said. "Moreover, this complement gene therapy can be applied to other complement-mediated muscle diseases as well."
Funding for this MDA grant began February 1, 2012.
Grantee: MG - Premkumar Christadoss, M.B.B.S.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2012 - MG — JianRong Sheng, Ph.D.

JianRong Sheng, assistant professor in the department of neurology and rehabilitation at the University of Illinois at Chicago Medical Center, was awarded an MDA research grant totaling $317,058 over three years. The funds will help support Sheng's study of potential therapeutic treatments for myasthenia gravis (MG).
MG is an autoimmune disease — a disease that occurs when the immune system attacks the body's own tissues. The attack occurs at theneuromuscular junction (NMJ), the space where signals pass between muscle and nerve.
Most MG occurs when the immune system uses special proteins calledantibodies to target either the acetylcholine (ACh) receptor, or muscle-specific kinase (MuSK), a protein that helps organize ACh receptors on the muscle cell surface.
"Current major therapies for MG produce general, nonspecific suppression of the immune system and are associated with significant long-term risk of infection and malignant tumors, and are therefore not optimal for treatment," Sheng explains. "There is a need to develop more focused treatments that only target those immune cells responsible for causing diseases without otherwise affecting the remainder of the immune system."
In previous studies, Sheng has shown that a growth factor called GM-CSF prevented development of a disease resembling MG called experimental autoimmune myasthenia gravis (EAMG). The treatment increases numbers of T cells and B cells, and Sheng showed that the T cells suppressed MG-associated antibodies.
Now, Sheng and colleagues are working on discerning the function, mechanisms and therapeutic potential of B cells in EAMG, and evaluating new medications aimed at mobilizing the cells for the treatment of MG.
Sheng's work potentially could change the fundamental approach to the treatment of MG from general, nonspecific immune suppression to focused, individualized and more specific therapy.
Funding for this MDA grant began Aug. 1, 2012.
Grantee: MG — JianRong Sheng, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2013 - DMD/BMD — Terence Partridge, Ph.D.

Terence Partridge, professor of integrative systemic biology and pediatrics at George Washington University and associate director of the Children’s Research Institute in Washington, D.C., was awarded an MDA research grant totaling $300,000 over a period of three years to investigate differences in muscle repair mechanisms in mice and humans, in the context of Duchenne muscular dystrophy (DMD).
When muscle is damaged, cells called satellite cells divide in order to replace the damaged muscle. This occurs in DMD, but eventually the satellite cell repair system is exhausted, leading to irreversible loss of muscle tissue. Mice also employ satellite cells, but the mouse system differs in some important ways from the human one, and these differences may have an impact on lessons that can be learned from using a mouse model of DMD (called the mdx mouse) to study repair in DMD.
Partridge will use the mdx mouse to investigate differences between satellite cell muscle building early and later in the mouse life cycle. “We now have ways of indelibly marking satellite cells during growth," Partridge says. "We can use this to ask whether the cells that are repairing the dystrophic muscle after two years of continuous repair are descended from the cells that were used for growth during the first three weeks of life, or whether they are recruited from other stem cell sources.
“At present, we do not know whether interventions designed to augment muscle regeneration that have been devised in the mdx mouse would be applicable to humans,” he says. “Design of effective strategies for rescuing or enhancing muscle repair in DMD requires that we learn which cells to use and how best to use them. We aim therefore to identify the significant participants and to characterize their relative roles in the formation, maintenance and repair of normal and chronically diseased muscle.”
Funding for this MDA grant began August 1, 2013.
Grantee: DMD/BMD — Terence Partridge, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Winter 2012 - Mito. Myopathy - Salvatore DiMauro, M.D.

MDA awarded a research grant totaling $272,974 over a period of two years to Salvatore DiMauro, the Lucy G. Moses Professor of Neurology at Columbia University Medical Center in New York. The funds will help support DiMauro’s research into developing a therapy designed to treat neutral lipid storage disease with myopathy (NLSDM), a mitochondrial myopathy.
NLDSM is a lipid storage myopathy. The genetic cause of the disease is mutations in the PNPLA2 gene, which carries instructions for ATGL, anenzyme (type of protein) that normally sticks to cellular “fat storage compartments” called lipid droplets and breaks down the more complex lipids, such as triglycerides, into simpler fatty acids. These fatty acids are then used by mitochondria — the “energy factories” that provide the power required for cells to perform their functions.
“We have been following for five years an 18-year-old woman with well-documented NLSDM, who is still totally asymptomatic although her muscle and cultured skin fibroblasts [immature cells that will develop into fibrous tissue] are loaded with triglyceride droplets,” DiMauro said. His team also has obtained cultured fibroblasts from four symptomatic patients with PNPLA2 mutations.
In cultured cells, DiMauro and colleagues plan to test the ability of compounds called beta agonists to reduce the abnormal triglyceride storage, with the ultimate goal of applying one of the strategies to their patients.
If favorable results are obtained, the investigators plan to conduct a one-person trial in their “pre-symptomatic patient,” followed by an international controlled trial.
Funding for this MDA grant began February 1, 2012.
Grantee: Mito. Myopathy - Salvatore DiMauro, M.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2013 - DMD/BMD — Lynn Megeney, Ph.D.

Lynn Megeney, senior scientist at the Sprott Centre for Stem Cell Research, Ottawa Hospital Research Institute in Ottawa, Ontario, Canada, was awarded an MDA research grant totaling $300,000 over a period of three years to study how muscle stem cells are controlled.
When muscles are damaged, stem cells called satellite cells divide to form new muscle. In muscular dystrophies such as Duchenne (DMD) andBecker (BMD) muscular dystrophies, satellite cells offer a pool of stem cells that, at least temporarily, can replace the muscle cells that are lost in the disease. However, Megeney notes, “the steps that control the self-renewal process of these cell types remain largely unknown.” He is investigating the role of a critical enzyme called caspase 3, which promotes maturation of satellite cells into muscle fibers. Caspase 3 targets and cleaves other proteins, either activating or destroying them, and thus altering cell development. Megeney has identified one such target, calledPax7, and will work to learn more about this system and to discover other caspase 3 targets, in both cell culture and mouse models of muscular dystrophy.
“We anticipate that the knowledge gained through our research efforts will provide a means to direct both the self-renewal process, as well as the maturation steps toward fully functional adult muscle cells,” Megeny says. “These basic science innovations will influence stem cell mediated therapies in general, impacting the range of dystrophies that are amenable to such a therapeutic intervention.”
Funding for this MDA grant began August 1, 2013.
Grantee: DMD/BMD — Lynn Megeney, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2013 - DMD — Steve Wilton, Ph.D.

Steve Wilton, foundation chair in molecular therapies at the Centre for Comparative Genomics at Murdoch (Australia) University, was awarded an MDA research grant totaling $300,000 over a period of three years to develop exon-skipping compounds for the less common mutations causingDuchenne muscular dystrophy (DMD).
Exon skipping has emerged as a promising therapeutic strategy for DMD. An exon is a section of a gene; multiple exons are combined to make the instructions for a protein. In DMD, a mutation that affects one or more exons of the dystrophin gene can prevent the protein from being made properly.
By blocking an exon or exons near the mutation, it is sometimes possible to restore the cell’s ability to create a smaller-than-normal, but still functional, dystrophin protein.
Different people carry different mutations and will require that different exons be blocked to treat those mutations. Experimental treatments that block (“skip”) exon 51 are now being tested in clinical trials, and results from early trials indicate these treatments may be beneficial.
But these treatments can only treat those who can benefit from blocking exon 51, Wilton points out, accounting for about 15 percent of all cases of DMD.
“Individuals with different mutations in their dystrophin gene need to have other morpholino oligos [the name for the exon-skipping treatment] designed to address their dystrophin mutations. Our project will extend exon skipping to the rare exons and other mutations that require multiple exons to be removed.”
The goal, he says, is to have these treatments ready to test if the current exon 51-targeting therapies continue to show promise in the clinic.
“If this therapy is shown to be able to slow or halt the progression of DMD, we want to have lead morpholino candidates ready to treat as many other DMD mutations as possible.”
Funding for this MDA grant began August 1, 2013.
Grantee: DMD — Steve Wilton, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2012 - MG — Feng Lin, Ph.D.

Feng Lin, associate professor in the department of pathology at Case Western Reserve University School of Medicine in Cleveland, Ohio, was awarded an MDA research grant totaling $390,000 over three years to study a potential new cell-based therapy for myasthenia gravis (MG).
MG is part of a large class of diseases known as autoimmune diseases, in which the body's immune system attacks its own tissues.
In MG, the attack occurs at the neuromuscular junction (NMJ) — the space where signals pass between muscle and nerve.
Symptoms include weakness in muscles that control the eyes, face, neck and limbs; partial paralysis of eye movements; double vision and droopy eyelids; and weakness and fatigue in the neck and jaws with problems in chewing, swallowing and holding up the head.
In previous work, Lin and colleagues developed a new method for generating a special group of cells that are highly effective at suppressing the immune system.
"Pilot studies in our labs found that this group of cells concurrently inhibited both T and B cell responses that lead to myasthenia gravis," Lin says.
Now Lin is testing the effectiveness of the special cells in treating MG in an animal model of the disease.
"Results from this project could help to develop these cells as a new, effective treatment for myasthenia gravis," Lin says.
Funding for this MDA grant began Aug. 1, 2012.
Grantee: MG — Feng Lin, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2013 - DMD — Rachelle Crosbie-Watson, Ph.D.
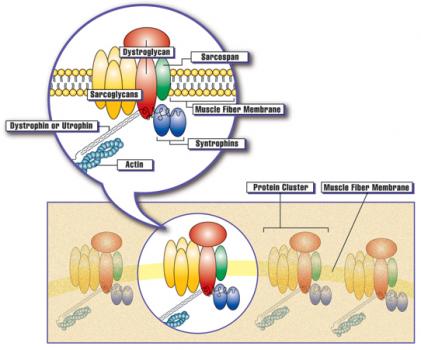
Rachelle Crosbie-Watson, professor of neurology at the University of California, Los Angeles, was awarded an MDA research grant totaling $300,000 over a period of three years to study whether increasing levels of the sarcospan protein can be therapeutic for Duchenne muscular dystrophy (DMD) and other muscle diseases.
In DMD, one of the major consequences of loss of dystrophin protein is that the muscle membrane, called the sarcolemma, becomes less stable and easier to damage. Replacing lost dystrophin is one therapeutic strategy; increasing sarcolemma stability through other means is an alternative.
“We have discovered a novel method that improves sarcolemma stability and adhesion [holding together],” Crosbie-Watson says, and the current study is aimed at testing the mechanisms and feasibility of this novel approach in animal models of DMD, limb-girdle muscular dystrophy (LGMD) and congenital muscular dystrophy (CMD).
The method involves delivering the gene that codes for the sarcospan protein. Raising the level of sarcospan leads to multiple effects at the sarcolemma, all contributing to increasing its stability. “We have shown that sarcospan ameliorates muscular dystrophy in the dystrophin-deficient mouse model for DMD, and we rationalize that sarcospan treatment will also benefit other forms of muscular dystrophy resulting from loss of muscle cell adhesion,” she says. The advantage of this approach is that, unlike dystrophin, the sarcospan gene is small and easily accommodated in the safest gene therapy vectors. Crosbie-Watson will continue fine-tuning this approach in the DMD mouse model, and extend it to models of LGMD and CMD.
“The outcome of these experiments will contribute to a better understanding of the molecular events contributing to the ability of sarcospan to alter expression of proteins at the cell surface,” she says, “and reveal the efficacy of sarcospan for the treatment of other muscular dystrophies.”
Funding for this MDA grant began August 1, 2013.
Grantee: DMD — Rachelle Crosbie-Watson, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Winter 2012 - IBM - Sanford Bernstein, Ph.D.

Sanford Bernstein, a professor of biology at San Diego State University in California, was awarded an MDA research grant totaling $370,311 over a period of three years. The funds will help support Bernstein's research into the underlying molecular causes of, and potential treatments for, inclusion-body myopathy type 3 (IBM-3).
IBM-3 is caused by a mutation in a protein called myosin, the "molecular motor" that drives muscle contraction.
"Our biochemical and ultrastructural studies have shown that IBM-3 myosin is prone to unfolding and to forming clumps called aggregates," Bernstein said. "Further, muscle appears to respond to the mutant protein by producing autophagosomes, cellular bodies designed to encapsulate and degrade protein aggregates."
Bernstein's team will study the components that comprise the IBM-3 aggregates. Then, in an IBM-3 fruit fly research model that they developed, the investigators will test various approaches aimed at hastening the clearance of protein aggregates and ameliorating defective muscle structure and function in IBM-3.
"IBM-3 is a rare disease, and no model that produces the inclusion bodies we see in our drosophila model has been developed," Bernstein said. "Hence, use of this model presents the current best opportunity to develop a better understanding of the basis of the disease or its possible treatment."
Funding for this MDA grant began February 1, 2012.
Grantee: IBM - Sanford Bernstein, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2012 - LGMD — Melissa Spencer, Ph.D.
Melissa Spencer, professor of neurology at the David Geffen School of Medicine at University of California, Los Angeles, was awarded an MDA research grant totaling $390,000 over three years to study the role of an enzyme called calpain 3 in type 2A limb-girdle muscular dystrophy (LGMD2A).
With colleagues, Spencer has demonstrated that characteristics and disease processes of LGMD2A are different from those seen in other dystrophies.
In Duchenne muscular dystrophy and other types of LGMD, the muscle cell membrane is weakened from the genetic mutation and the muscle cells die, Spencer explains. "In LGMD2A, muscle membranes remain intact, a finding which has left researchers without an explanation for the underlying mechanism of disease."
LGMD2A is caused by mutations in the CAPN3 gene, which carries genetic instructions for building a protein called calpain 3. Over the last decade, Spencer's team has generated numerous research mouse models that have provided insight about the biological function of calpain 3 in muscle development and muscle growth, but it's remained unclear how CAPN3 mutations lead to muscle dysfunction.
The team discovered that muscles lacking calpain 3 have severe deficits in the signaling pathways responsible for muscle growth — particularly thecalcium calmodulin kinases (CaMK) pathway.
Now Spencer is working on determining the relationship between calpain 3 and CaMK signaling.
Spencer's work could uncover the underlying defects for LGMD2A and lead to therapeutic targets for stimulating muscle growth in the setting of muscle degeneration. It also could shed light on other related muscular dystrophies, such as multiminicore disease.
Funding for this MDA grant began Aug. 1, 2012.
Grantee: LGMD — Melissa Spencer, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2013 - DMD — Morayma Reyes, M.D., Ph.D.
Morayma Reyes, assistant professor of pathology at the University of Washington in Seattle, was awarded an MDA research grant totaling $300,000 over a period of three years to study a strategy for reducing heart muscle damage in Duchenne muscular dystrophy (DMD).
People with DMD develop cardiovascular problems in their early teen years, and cardiac complications are the major cause of death in the disease. A significant contributor to the damage done to heart muscle is fibrosis, or development of fibrous tissue within the muscle. Fibrosis is a response toinflammation, part of the body’s immune response. Reyes will be studying the effect of blocking a molecular pathway that contributes to that inflammation.
She has characterized cells surrounding blood vessels within the fibrotic tissue of heart muscle in the mdx mouse, a mouse model of DMD. “We hypothesize that these cells respond to inflammatory cues and deposit collagen, and thus form fibrotic tissue in the perivascular tissue of mdx hearts,” she says. “We will study the role of these cells in mdx cardiac fibrosis.”
In particular, she will study a potentially important signaling pathway, called the PDGF receptor alpha pathway. “We hypothesize that signaling through PDGF receptor alpha results in activation of a fibrotic program in these cells. We will test this by administration of PDGF-A, which should result in increased fibrosis.”
She will then block the pathway to reduce fibrosis, testing the potential benefits of a drug called Crenolanib. “These studies will lay the foundation for preclinical studies using Crenolanib to treat fibrosis in DMD patients,” Reyes says.
Funding for this MDA grant began August 1, 2013.
Grantee: Morayma Reyes, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Winter 2012 - FSHD - Scott Harper, Ph.D.

MDA awarded a research grant totaling $317,464 over a period of three years to Scott Harper, an assistant professor of pediatrics at the Ohio State University College of Medicine in Columbus. The funds will help support Harper's work to develop a new mouse model of facioscapulohumeral muscular dystrophy (FSH, or FSHD).
"The root causes of FSHD have puzzled scientists and clinicians for decades, but some recent breakthroughs support the hypothesis that a gene called DUX4 is involved in the disease," Harper said. "Now that we have a gene target in DUX4, we can begin developing treatments that counteract that gene's toxic effects to muscle."
Harper noted that animal models of human diseases including FSHD are "important tools for testing therapeutics," but that "unfortunately, no such model exists for FSHD."
With colleagues, Harper plans to develop an FSHD research mouse model that contains DUX4 for use in the study of the disease, and the development and testing of potential therapies.
"I am hopeful that the FSHD field has turned a corner and that we have now entered a new era in which rational therapeutic strategies for FSHD are now possible," Harper said. "We believe this animal model will help in this effort."
Funding for this MDA grant began February 1, 2012.
Grantee: FSHD - Scott Harper, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2012 - FSHD — Rossella Tupler, M.D., Ph.D.
MDA awarded a research grant totaling $260,000 over two years to Rossella Tupler, research assistant professor in the program of molecular medicine at the University of Massachusetts Medical School in Worcester. The funds will help support Tupler’s search for the molecular cause of facioscapulohumeral muscular dystrophy (FSHD).
FSHD has been associated with a reduction (eight or fewer) in the number of D4Z4 elements (alleles) and the subsequent expression (gene activity) of DUX4, Tupler explains. “However, studies of families in Italy, Brazil and the United States suggest that D4Z4 reduction and DUX4 expression are not sufficient to cause disease.”
In Italian families, a study of 253 unrelated people with FSHD revealed that 204 (80.6 percent) carry D4Z4 alleles with 1-8 units, 19 (7.5 percent) have D4Z4 alleles with 9-10 repeats, and 30 (11.8 percent) carry D4Z4 alleles with 11 repeats or more.
In the United States, family members with identical D4Z4 repeat lengths included members who did not have the disease and members with classical FSHD; not all had DUX4 activity.
“Collectively, these results indicate that those DNA anomalies are not sufficient to cause FSHD and additional factors are necessary to disease development,” Tupler says.
With colleagues, Tupler is working to identify genetic elements that can cause FSHD disease onset. The team is using a technique called whole-genome sequencing to study the DNA of 12 families from Italy, Brazil and the United States in which D4Z4 reduction does not correlate with the presence of disease.
“A profound rethinking of the genetic disease mechanism and modes of inheritance of FSHD is now required,” Tupler says, “and entirely new models and approaches are needed.”
Funding for this MDA grant began Aug. 1, 2012.
Grantee: FSHD — Rossella Tupler, M.D., Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Winter 2012 - FSHD - Charles Emerson, Ph.D.

MDA awarded a research grant totaling $600,000 over a period of three years to Charles Emerson, director and senior scientist at Boston Biomedical Research Institute in Watertown, Mass. The funds will help support Emerson’s efforts to identify genetic modifiers of the DUX4 gene; such modifiers potentially could become therapeutic targets in facioscapulohumeral muscular dystrophy (FSH, or FSHD).
Evidence from recent studies suggests that the DUX4 gene (which carries instructions for the DUX4 protein) is the primary underlying molecular cause of FSHD. However, the mechanism by which DUX4 causes the disease remains unclear.
Preliminary studies conducted in Emerson’s lab, involving a large group of FSHD-affected families, have confirmed the importance of the DUX4 gene as a factor in the FSHD disease process. The studies also have identified an alternative disease mechanism in which family-specific genetic modifiers modulate DUX4 function and gene expression (production of the DUX4 protein), effectively enhancing or suppressing disease signs, symptoms and severity.
Emerson’s team plans to identify the underlying mechanisms that modulate DUX4 via studies in FSHD-affected families. Once identified, the DUX4 gene disease modifiers potentially may serve as therapeutic targets for treatment of FSHD.
Funding for this MDA grant began February 1, 2012.
Update (Feb. 12, 2014): Charles Emerson has relocated to the University of Massachusetts Medical Center in Worcester.
Grantee: FSHD - Charles Emerson, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2012 - FSHD — Joel Chamberlain, Ph.D.

Joel Chamberlain, research assistant professor in the department of medicine at the University of Washington in Seattle, was awarded an MDA research grant totaling $330,780 over three years to study a therapeutic approach called RNA interference (RNAi) for the treatment of facioscapulohumeral muscular dystrophy (FSHD).
"Despite the surprising complexity of the FSHD disease pathway, several lines of research converge to identify DUX4 protein production as the final step in the disease pathway," Chamberlain says.
Now, Chamberlain and colleagues are working to develop a therapy that targets production of DUX4 through interaction with DUX4 messenger RNA, or mRNA. (RNA is a chemical cousin to DNA and is involved in a chain of steps between DNA and cellular protein production.)
"To carry out RNAi, we will use an RNA that specifically binds to the DUX4 mRNA and directs the cell to destroy the target DUX4 mRNA," Chamberlain explains. "Once inside the muscle, the RNA made to carry out RNAi is produced continuously to treat disease and is expected to remain active for many years."
The team also is working to create a mouse model of FSHD that mimics the human disease. The investigators will use the model — as well as new cell and mouse models from collaborating laboratories — to test their DUX4 RNAi approach. They plan to make the model available to other researchers for the testing of additional potential therapies.
"We have developed this targeted approach, referred to as RNA interferenceor RNAi, to reverse disease," Chamberlain says. "It works well in mouse models of muscular dystrophies, and we are the only laboratory to have succeeded in changing the course of the disease by delivering this therapy to all the muscles of the mouse with a single intravascular (into a blood vessel) injection."
Funding for this MDA grant began Aug. 1, 2012.
Grantee: FSHD — Joel Chamberlain, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Winter 2012 - DMD/BMD - Pura Muñoz-Canoves, Ph.D.

MDA awarded a research grant totaling $390,000 over a period of three years to Pura Muñoz-Canoves, ICREA research professor and cell biology coordinator at Pompeu Fabra University in Barcelona. The funds will help support Muñoz-Cánoves' research into strategies aimed at reducing muscle scarring (fibrosis) in people with Duchenne (DMD) and Becker (BMD)muscular dystrophies.
Fibrosis is a hallmark of DMD and a major factor in gauging disease severity. Fibrosis also compromises the efficacy of ongoing preclinical gene- and cell-delivery therapies. No treatment exists for reversing fibrosis in DMD; nor is there a clear understanding of the mechanisms underlying fibrosis development in dystrophic muscle (muscle that is deficient in dystrophin protein).
Muñoz-Canoves and colleagues plan to test whether and how a protein called PAI-1 (plasminogen activator inhibitor-1) may regulate inflammation-driven muscle degeneration and fibrosis development in muscular dystrophy.
"The discovery of new causes of fibrosis development in dystrophic muscle will provide an opportunity for new strategies to halt disease progression," Muñoz-Canoves said. "Since muscle fibrosis also represents a major obstacle for successful engraftment of stem cells in dystrophic muscle, targeting molecules promoting fibrosis appears to be an easy-to-test alternative to improve future DMD stem cell therapies.
Funding for this MDA grant began February 1, 2012.
Grantee: DMD/BMD - Pura Muñoz-Canoves, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2012 - EDMD/LGMD/CMT — Yosef Gruenbaum, Ph.D.

MDA awarded a research grant totaling $300,009 over three years to Yosef Gruenbaum, professor and elected chairman at the Alexander Silberman Institute of Life Sciences, Hebrew University of Jerusalem, in Israel. The funds will help support Gruenbaum’s study of proteins called lamins and their role in muscle diseases such as Emery-Dreifuss (EDMD) and limb-girdle (LGMD) muscular dystrophies, and Charcot-Marie-Tooth disease (CMT).
Relatively little is known about the biological pathways that are affected in muscle cells that have lamin defects, and why these changes lead to muscular dystrophy, Gruenbaum says. Now, using a nematode (worm) model of EDMD, Gruenbaum and colleagues are studying those pathways.
The investigators will use a variety of techniques in structural biology, genetics, cell biology and live imaging to investigate the effects of EDMD-linked lamin mutations and determine how those mutations affect the normal health and development of muscles.
Gruenbaum’s work is expected to uncover the underlying mechanisms at work in EDMD and potentially could lead to the identification of new drug targets and the development of new therapies to treat EDMD.
Funding for this MDA grant began Aug. 1, 2012.
Grantee: EDMD/LGMD/CMT — Yosef Gruenbaum, Ph.D.
Grant type:
Award total:
Institution:
Country:
MDA Resource Center: We’re Here For You
Our trained specialists are here to provide one-on-one support for every part of your journey. Send a message below or call us at 1-833-ASK-MDA1 (1-833-275-6321). If you live outside the U.S., we may be able to connect you to muscular dystrophy groups in your area, but MDA programs are only available in the U.S.